Accuracy


What is ISO 15197?
ISO 15197 is the standard for blood glucose monitoring that MUST be fulfilled in order to apply a CE mark to a BGM system as part of any route to EC certification.
Why are there different versions of ISO 15197?
The first ISO 15197 version was published and harmonised in 2003 and is therefore known as “ISO 15197:2003” AND “EN ISO 15197:2003”. The “EN” refers to the UK version in English of the version harmonised across Europe.
In 2013 a new version was published (“ISO 15197:2013”). However, this standard was not harmonised across Europe until 2015. The new UK harmonised version is known as “EN ISO 15197:2015” (based on the ISO 15197:2013 published standard).
Therefore, EN ISO 15197:2015 supersedes EN ISO 15197:2003.
How do you know if a meter complies?
-
Ask to see a “Declaration of Conformity” Certificate
Blood glucose monitoring systems in conjunction with their strips will have a “Declaration of Conformity” Certificate to demonstrate their compliance if they have undergone and passed a conformity assessment.
What is conformity assessment? Conformity assessment involves a set of processes that demonstrate a product, service or system meets the requirements of the standard by that Notified Body.
Undergoing and passing the conformity assessment process will result in certification which provides consumers and other stakeholders with added confidence and helps regulators ensure that health, safety and environmental conditions are met.
-
Ask to see the unique EC certificate issued by the Notified Body referenced on the declaration of conformity.
Every blood glucose monitoring system that has been through the above testing will be issued an EC certificate.
Without evidence of these certificates, conformity to EN ISO 15197:2015 cannot be assured.
Our Conformance
AgaMatrix Europe is pleased to confirm that both WaveSense JAZZ™ meters exceed the full requirements of EN ISO 15197:2015.
The Declaration of conformity and EC Certificate which confirms conformity for our blood glucose monitoring systems, test strips, and control solution are available here, along with our ISO Whitepapers and independent ISO study.
Are the AgaMatrix WaveSense JAZZ family of meters affected by Haematocrit?
Haematocrit is the percentage of red blood cells in the blood. This varies from person to person and in individuals from time to time. Blood Glucose Monitoring Systems that use electrochemistry to measure glucose tend to be affected by haematocrit levels in the blood sample. This is because the diffusion of molecules in the blood is slower when haematocrit is high and faster when haematcrit is low.1
WaveSense is a suite of patented technologies that uniquely applies Dynamic Electrochemistry in blood glucose measurement to detect and correct for common sources of error, including haematocrit, and so, providing accurate results.
Haematocrit does not significantly affect Wavesense JAZZ readings2
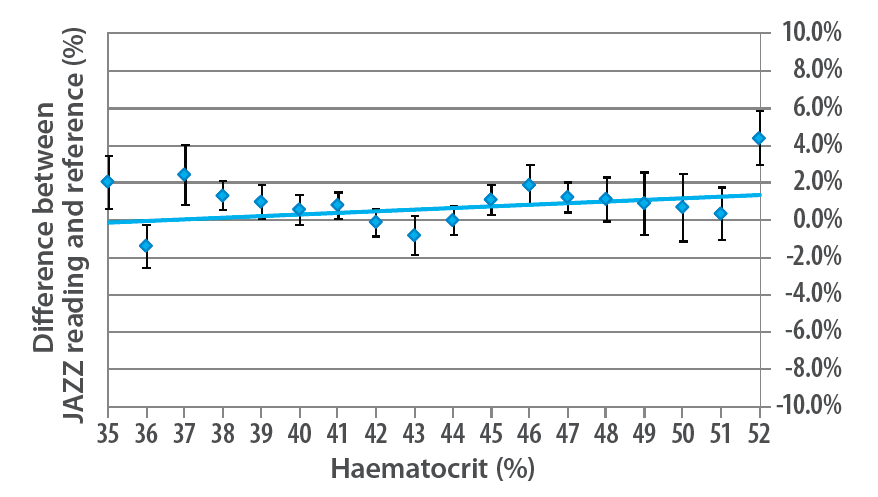
Figure 1 shows how WaveSense JAZZ readings compare to the reference result for each haematocrit value.
Based on this analysis, blood samples with a haematocrit of 52 are predicted to give readings only 1.5% higher than those with a haematocrit of 35. (i.e. a glucose level of 5.0 mmol/L would be recorded as 5.1 mmol/L). This difference is of no clinical significance.
To view our paper in full on the study that indicates this please click on the link below:
Use in Pregnancy
Haematocrit can change rapidly during pregnancy. Because accuracy is not significantly affected by varying haematocrit when testing blood glucose levels using the JAZZ meters, increasing numbers of gestational departments within UK hospitals are now giving the WaveSense JAZZ blood glucose meters to patients with gestational diabetes.
2Iyengar, S. Et al. WaveSense Algorithms Account for Haematocrit while Determining Blood Glucose. Poster, 45th Annual Meeting of the European Association for the study of Diabetes (EASD). 2009
